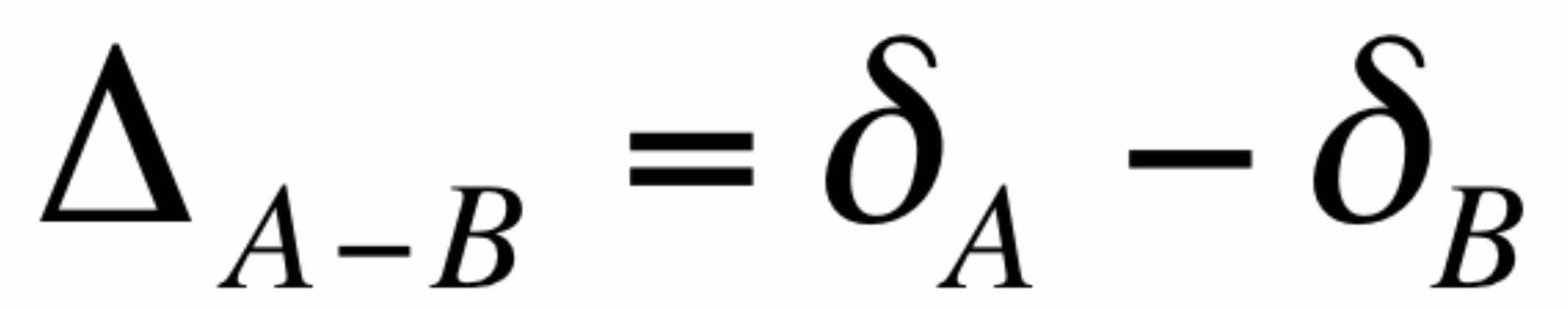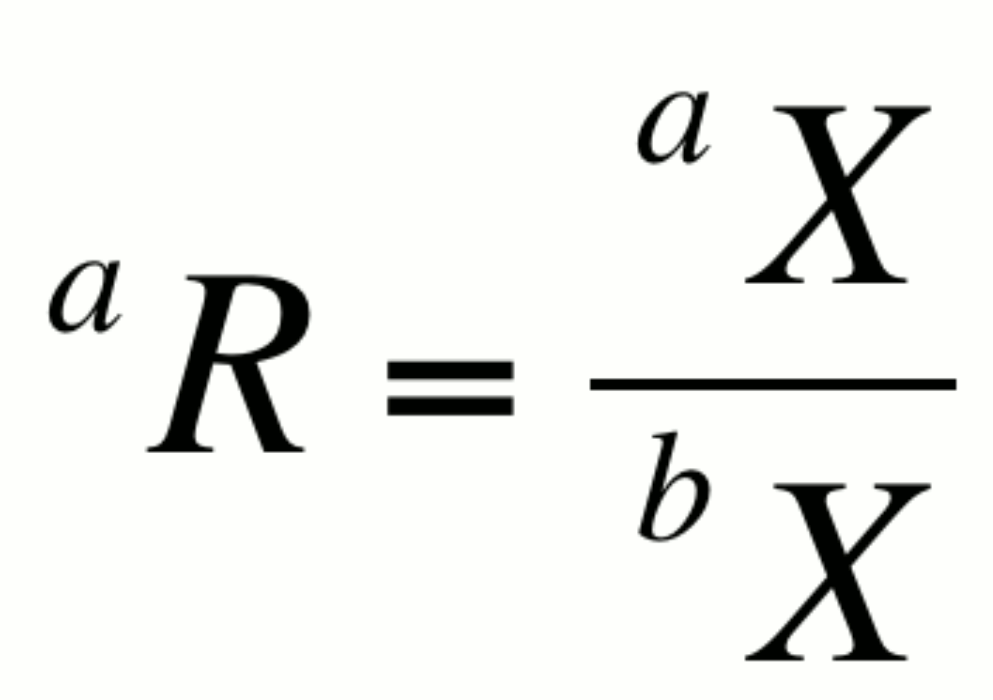
A Primer on Isotope Notation
The isotope ratio:
The relative abundance of two isotopes a and b of element X can be expressed as an isotopic ratio, aR:
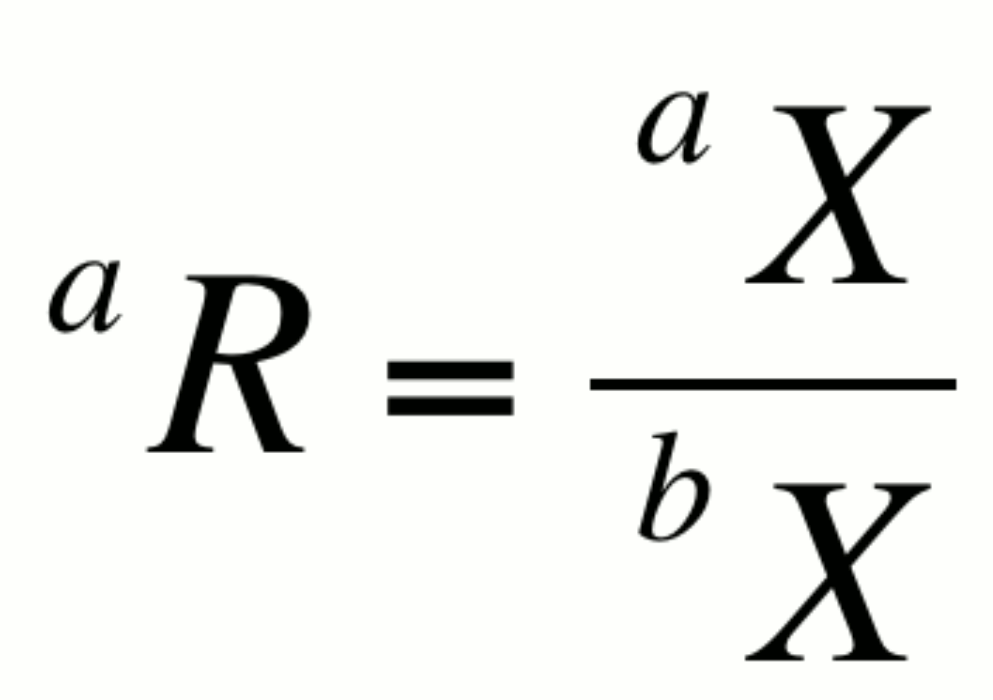
By convention, the more abundant isotope is placed in the denominator. This notation is not particularly helpful in itself however, as changes in R could result from changes in either aX or bX. It is however the basis for several expressions that are very useful.
Fractional abundance, aF:
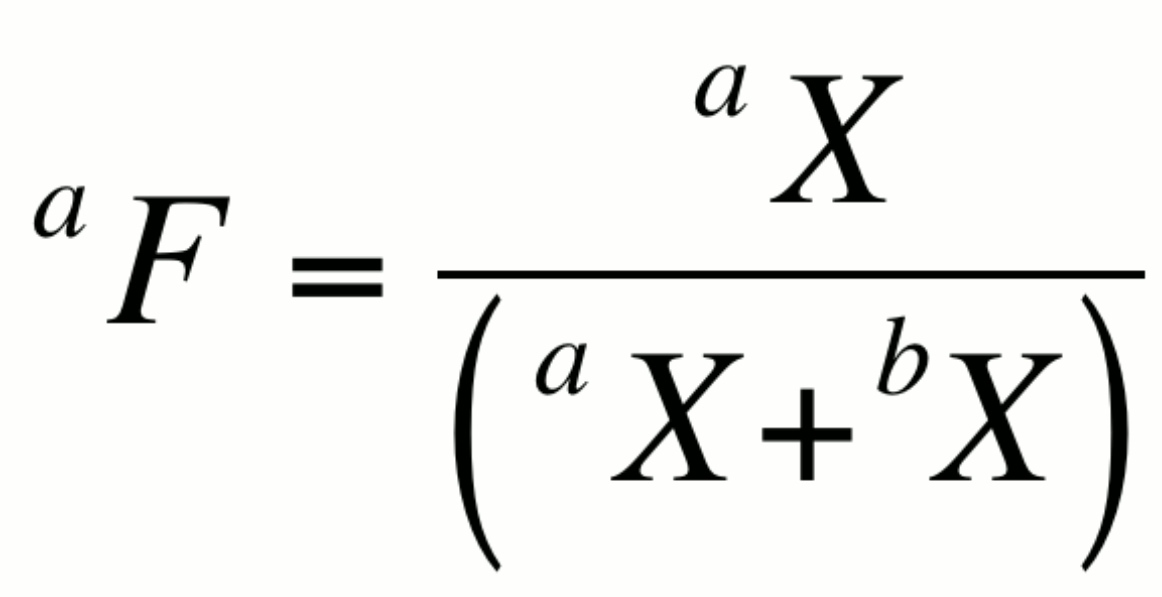
This expression is particularly useful in artificially enriched
systems where the ratio of aX to bX is
increased by the intentional addition of a pure source of one of
the two isotopes (usually the heavy isotope). The pure
source is referred to as a spike, the process of adding the
source as "spiking" a sample. Spikes allow us to monitor the
movement of isotopes from one reservoir to another in response
to one of more chemical reactions.
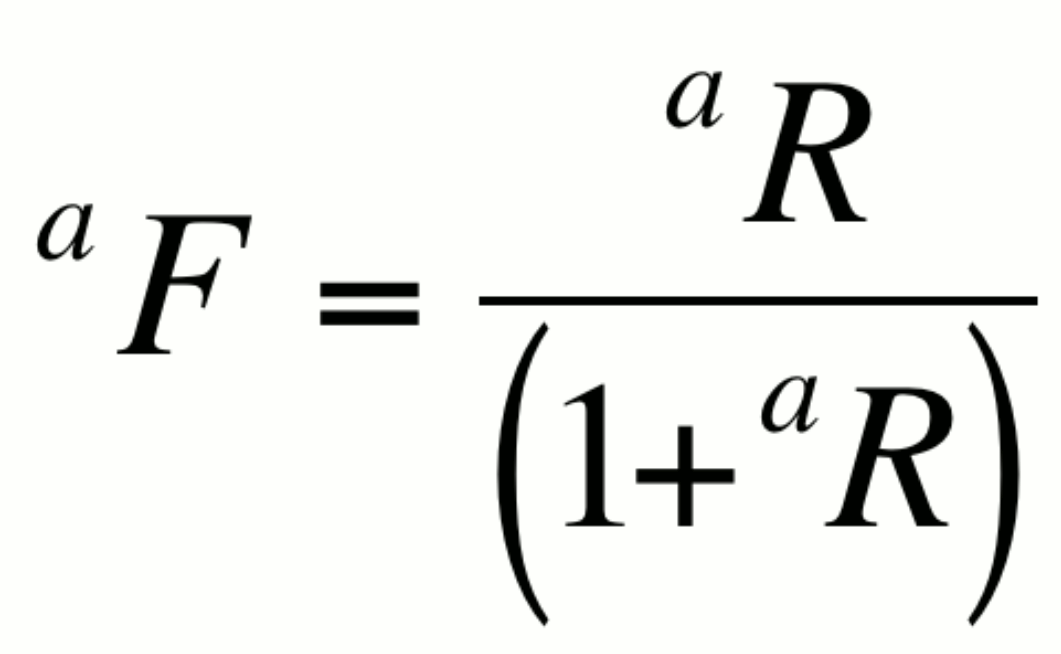 or alternatively,
or alternatively, 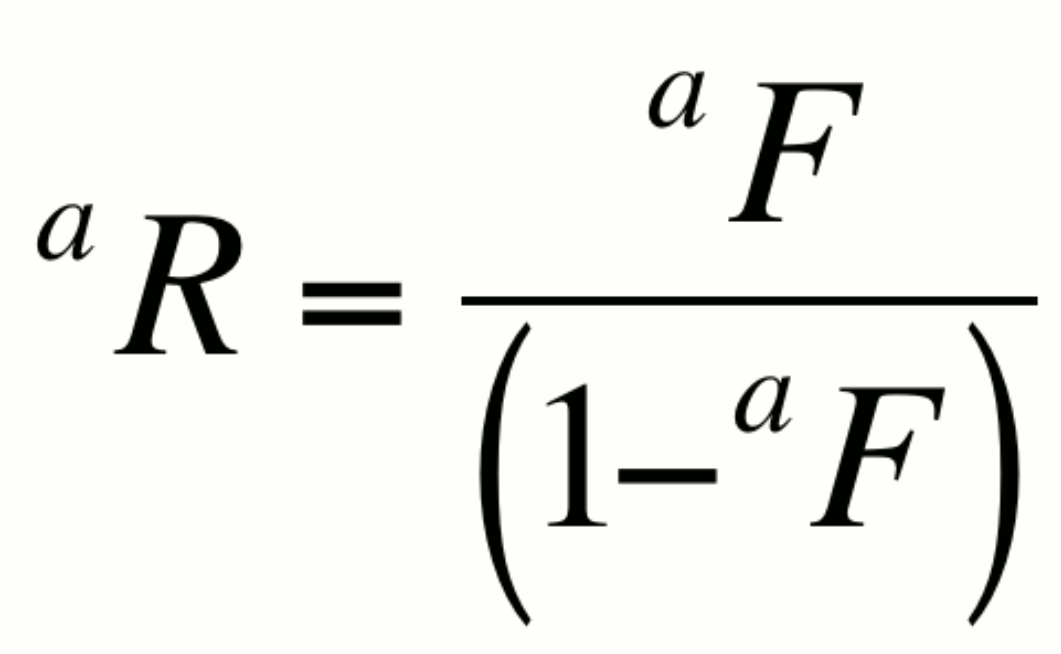
The delta notation, daX.
In many natural systems, the isotopic ratio aR exhibits variability in the range of the third to fifth decimal place. Numbers this small are best presented in terms of per mil, or parts per thousand (‰). The ratio in a sample, denoted here as aRx can be expressed relative to the isotopic ratio of a standard aRstd using a difference relationship knows as the delta notation (daX):
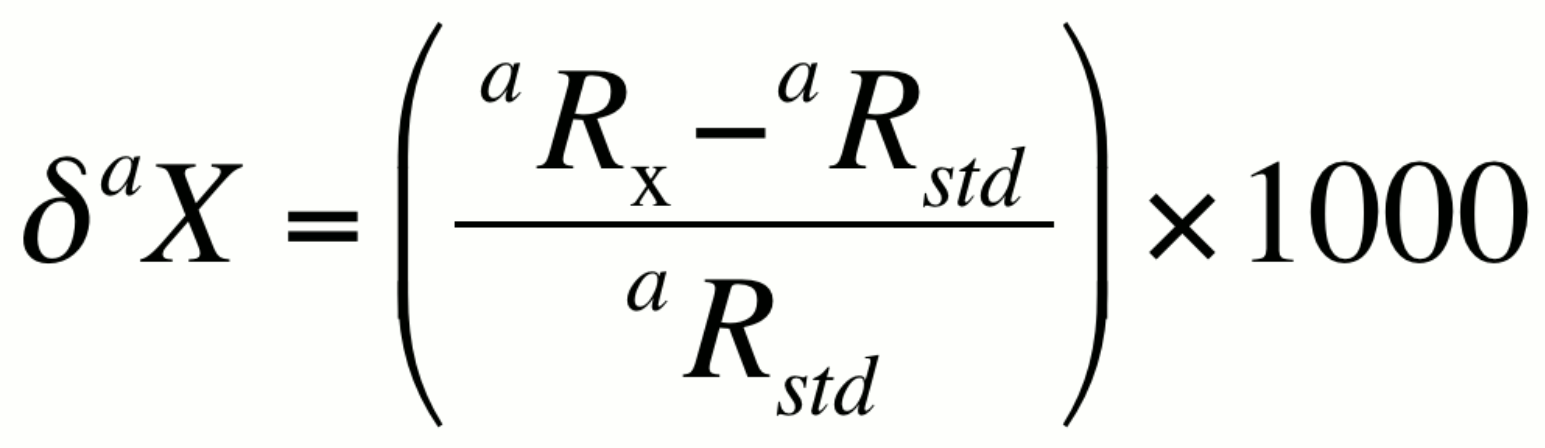 or
alternatively,
or
alternatively, 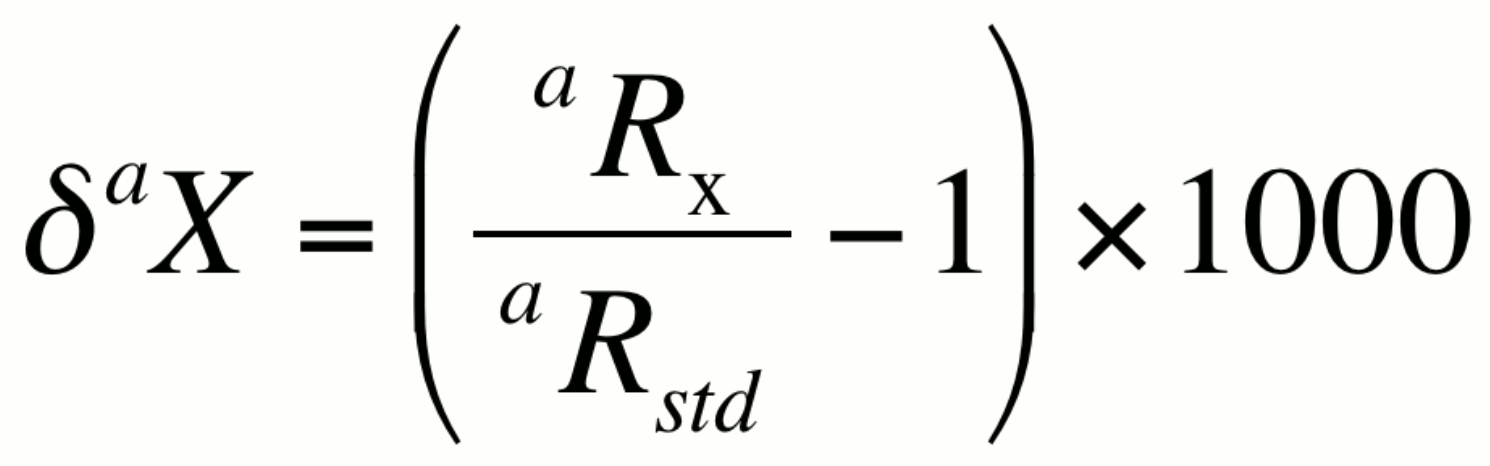
Change of isotopic reference scale:
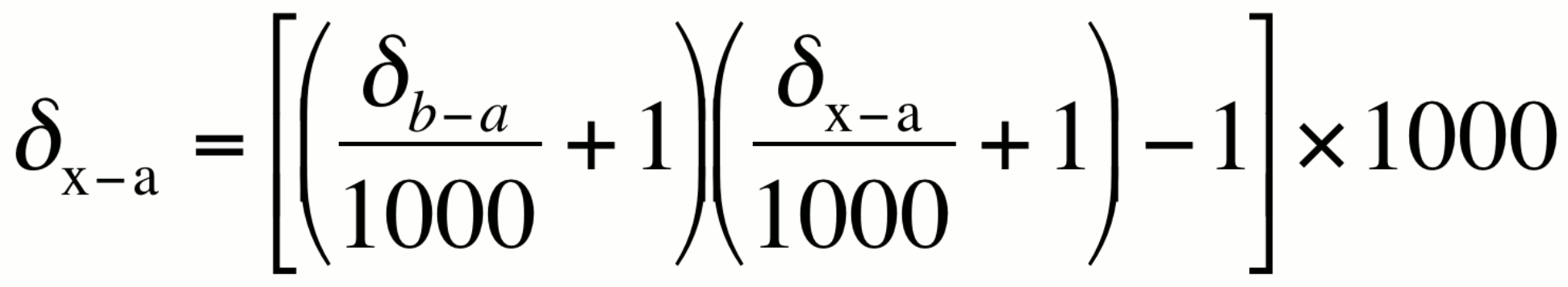
This relationship can be used to convert an isotopic value from one reference scale to another reference scale.
Measures of fractionation: a, 1000ln(a), e, and D
We are interested in measuring the isotopic offset between substances. Such offsets arise from the expression of an isotope effect due to equilibrium or kinetic fractionation during a physical process or chemical reaction. The size of this isotopic fractionation can be expressed in several ways.
The fractionation factor a is
defined as:
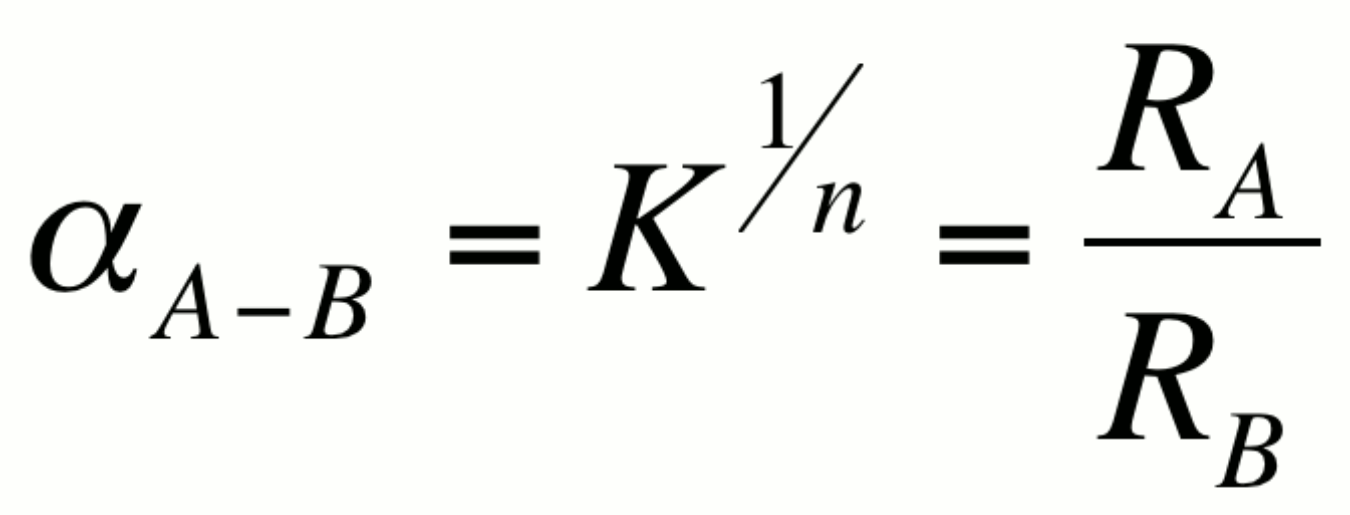
where K is the equilibrium constant for the associated reaction
and n is the number of atoms exchanged. For simplicity, isotope
exchange equations are usually written such that n=1 so that
a =K. It
is worth noting that most values of a are close to 1, with
variability in the third through fifth decimal place. We
can see that this is the case if we express a in terms of the
delta notation
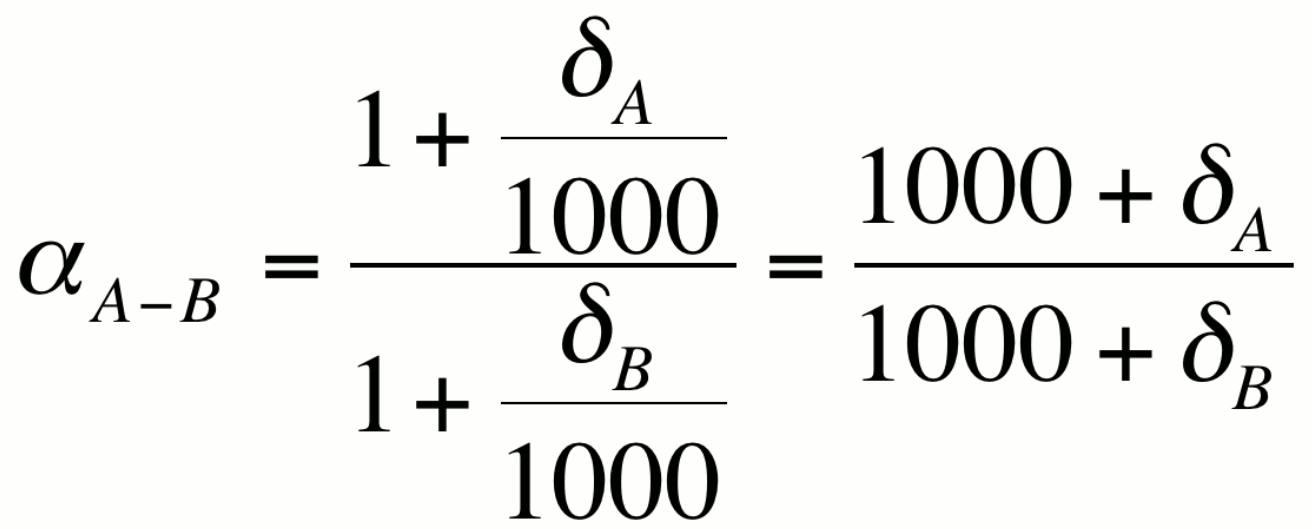
Because a is close to unity, it is convenient to express fractionation in ways that accentuate the differences between dA and dB. This can be done in one of three ways which yield approximately the same value for the per mil fractionation.
Natural log notation, 1000ln(a):
One would think that this notation would have its
own symbol, but surprisingly, it does not! We can approximate
the fractionation in per mil from the fractionation factor a by
taking the natural log of a and multiplying it by 1000. This is
written simply as 1000ln(a). Mathematically, this works
because we can think of a as being composed of 1 + e, a small
deviation. Now if we take the natural log of 1+ e, the result
can be expanded as a type of infinite series known as a
Maclaurin series in which the first term e, is the largest and
thus serves as a reasonable approximation of the natural log of
a. If we retain more terms, we would obtain a more accurate
result, but by convention, only the first term is retained:
Multiplying by 1000 yields the result
in per mil. But since we are interested in e
anyway, there is an easier way to express the per mil
fractionation.
The epsilon notation, e:
The epsilon notation has the advantage over the 1000ln(a)
notation in that it is an exact expression of the per mil
fractionation. There is a final way of determining the per mil
fractionation. This last method is the least accurate, but most
commonly applied because of its simplicity.
The capital delta notation, D: