Earth's Radiant Energy Balance and Oceanic Heat Fluxes
How do greenhouse gases influence earth's surface temperature?
Earth's average surface is 32°C warmer than it would be if it had no atmosphere. A planet the size of earth at earth's distance from the sun, and in thermodynamic equilibrium with solar energy (sunlight), would have an average surface temperature of -18°C. Earth's mean, global surface temperature for the period 1901 to 2000 is 13.9°C, which is 32°C warmer. This increase in temperature is due to greenhouse gases in earth's atmosphere.
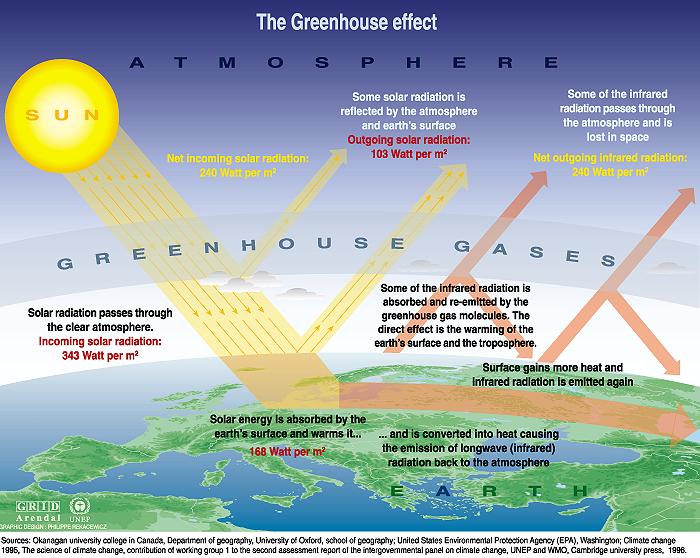
The greenhouse effect. From the Introduction to Climate Change written by the United Nations Environmental Program's UNEP Global Resources Information Database (GRID) office in Arendal Norway.
How does the greenhouse effect work? To begin, view the BBC animation illustrating the Greenhouse Effect. The basic idea is: the atmosphere is transparent to solar radiation. It allows sunlight to reach and warm the earth's surface. The atmosphere is mostly opague to infrared radiation emitted from earth's surface, hindering the emission of radiation from the surface to space, keeping the surface warm.
Now, let's discuss the details of how greenhouse gases warm earth's surface. They are:
- Sunlight reaches earth. It has an intensity of 1360 W/m2, and the average over all the earth is 343 W/m2. Remember, the average includes day and night, from the equator to the poles. Most solar energy has a wavelength close to 0.5 µm.
- 49% of the incoming sunlight goes straight through the atmosphere and it is absorbed by earth's surface, mostly in the tropical ocean.
- 31% of the incoming sunlight is reflected back to space, 22% by clouds, and 9% by the surface.
- The remaining 20% is absorbed in the atmosphere.
- Sunlight that is absorbed by earth's surface and atmosphere warms the surface and the atmosphere.
- The surface cools primarily in two ways:
- All surfaces radiate heat, mostly at wavelengths close
to 10 µm wavelength. On average, they radiate 390 W/m2.
This is more than the incoming solar heat, and earth would rapidly
cool if there were no greenhouse gases.
- 90% of the infrared energy emitted by the surface is absorbed by greenhouse gases in the atmosphere.
- 10% of the infrared energy emitted by the surface goes directly to space, mostly in polar regions.
- The ocean evaporates, losing latent heat. On average, two meters of water is evaporated from the tropical ocean each year. In addition, small amounts of water evaporate from land and plants on land. On average 78 W/m2 is lost by evaporation. All latent heat is released in the atmosphere when the evaporated water condenses as water in clouds and rain. This warms the atmosphere.
- All surfaces radiate heat, mostly at wavelengths close
to 10 µm wavelength. On average, they radiate 390 W/m2.
This is more than the incoming solar heat, and earth would rapidly
cool if there were no greenhouse gases.
- The atmosphere cools by radiating infrared energy to space.
- 45% of the heat that warms the atmosphere is radiated to space (235 W/m2).
- 55% of the heat that warms the atmosphere is quickly re-radiated radiated back to the earth (324 W/m2). This warms the earth and the lower atmosphere.
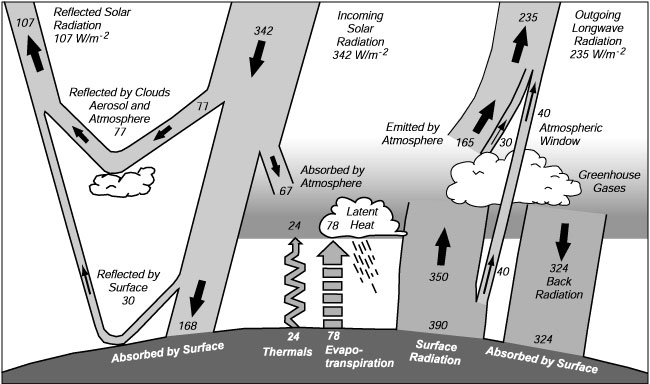
Thus greenhouse gases absorb radiant energy from earth's surface, and reradiate most of it back to the surface, keeping the surface warm. If there were no greenhouse gases, the surface would rapidly radiate heat away to space. The figure below shows these values a little more clearly.
The mean annual radiant energy and heat balance of the Earth. From Houghton et al., (1996: 58), which used data from Kiehl and Trenberth (1996).
Please note the special use of words. Sunlight is reflected by surfaces and absorbed by gases and surfaces. Greenhouse gases do not reflect sunlight. Infrared energy is emitted and absorbed by surfaces and greenhouse gases. Radiation refers to radiant energy, not nuclear radiation.
Notice also that the amount of infrared energy emitted at the top of the atmosphere (235 W/m2) must equal almost exactly the amount of solar energy absorbed by earth (342–107 W/m2). The small difference, about a watt per square meter, leads to global warming or cooling.
Greenhouse Gases
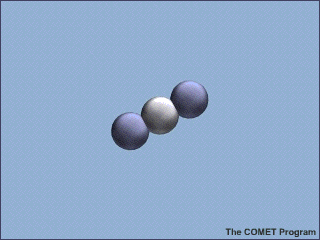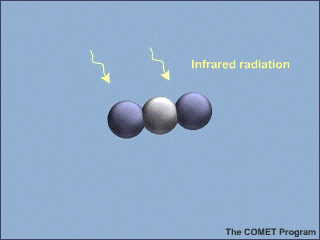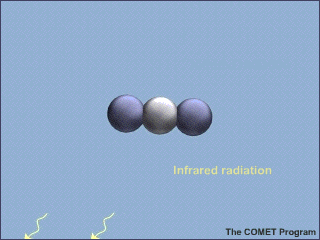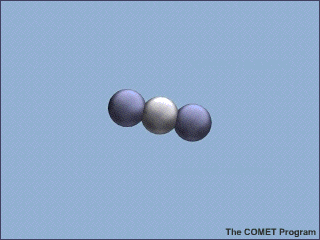
Greenhouse gases are composed of molecules with three or more atoms that absorb infrared energy but not visible light. There are four important greenhouse gases, and several less important gases. This animation, from the University Corporation for Atmospheric Research shows a tri-atomic, CO2 molecule absorbing and re-emitting a photon of infrared energy. Remember, molecules can absorb or emit only photons with precisely the right amount of energy, and energy is inversely proportional to wavelength.
Animated image of molecule absorbing and emitting radiation. Absorption of an infrared photon increases the energy of the molecule, shown here as a vibration of the position of the oxygen atoms relative to the carbon atom in the molecule. The molecules emits a photon of exactly the same energy that it absorbed, and stops vibrating.
From UCAR web page on the greenhouse effect.
- Water vapor H2O is by far the most important greenhouse gas. It accounts for about 30°C of the 36°C increase in earth's surface temperature due to the combined influence of all greenhouse gasses.
- Carbon dioxide CO2 is the second most important greenhouse gas. Its lifetime in the atmosphere hard to determine, but is in the range of 5 to 100 years.
- Methane CH4. Methane is a reactive trace gas (lifetime
 10 yr) that plays
key roles in Earth’s climate and atmospheric chemistry.
Its infrared activity currently accounts for
10 yr) that plays
key roles in Earth’s climate and atmospheric chemistry.
Its infrared activity currently accounts for  12% of enhanced greenhouse forcing, and it participates
in tropospheric O3 and OH regulation and in the stratospheric H2O
cycle. Tropospheric CH4 has more than doubled during
the past 200 yr
and the
current tropospheric rate of increase is both significant and variable,
reflecting varying source-sink strengths (Naqvi, 2005).
12% of enhanced greenhouse forcing, and it participates
in tropospheric O3 and OH regulation and in the stratospheric H2O
cycle. Tropospheric CH4 has more than doubled during
the past 200 yr
and the
current tropospheric rate of increase is both significant and variable,
reflecting varying source-sink strengths (Naqvi, 2005). - Nitrous oxides N2O. Unlike CH4, the atmospheric
budget of N2O is greatly influenced by its production in
the ocean and exchange across the air–sea interface, since the
oceans are estimated to account for at least one-third (4.7–6.3
Tg N2O y−1) of N2O inputs to
the atmosphere from all natural sources (
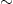 15
Tg N2O y−1) (Naqvi, 2005).
15
Tg N2O y−1) (Naqvi, 2005). - Less important gases are tropospheric ozone O3 and a number of substances containing fluorine, among them HFCs (compounds of hydrogen, fluorine and carbon).
The net effect of gases in the atmosphere is to allow sunlight in, and to hinder the transfer of heat from the surface to space. Absorption of radiant energy by atmospheric gases is a function of wavelength, and this is important to understanding how different molecules trap radiant energy.
This plot shows the transparency of the atmosphere as a function of wavelength (bottom) and the influence of absorption by different atmospheric gases. Between 0.6 µm and 0.3 µm the atmosphere is clear (an atmospheric window) allowing sunlight to reach the surface. There is a less clear window near 10 µm that allows infrared energy to carry heat from the surface to space. Greenhouse gases partly obstruct the clarity of the window, helping keep the surface warm. Note that water vapor is highly variable, absorbing infrared energy much more strongly in the tropics and much less in the polar regions.
Plot from from GEOG-1425 class notes, Katherine Kink, University of Minnesota.
The Role of Water and Water Vapor in the Earth System
The influence of greenhouse gases is only part
of the process.
If the earth were in radiative equilibrium, with an atmosphere, the surface
temperature would be 67 degrees C. This does not happen because water
evaporates from the surface, mostly from tropical seas, cooling the surface
(Philander, 1998: 78).
The simple picture of the greenhouse mechanism is seriously oversimplified. Many of us were taught in elementary school that heat is transported by radiation, convection, and conduction. The above representation [of the simple greenhouse effect] only refers to radiative transfer. As it turns out, if there were only radiative heat transfer, the greenhouse effect would warm the Earth to about seventy-seven degrees centigrade rather than to fifteen degrees centigrade. In fact, the greenhouse effect is only about 25 percent of what it would be in a pure radiative situation. The reason for this is the presence of convection (heat transport by air motions), which bypasses much of the radiative absorption ... The surface of the Earth is cooled in large measure by air currents (in various forms including deep clouds) that carry heat upward and poleward. One consequence of this picture is that it is the greenhouse gases well above the Earth's surface that are of primary importance in determining the temperature of the Earth. That is especially important for water vapor, whose density decreases by about a factor of 1,000 between the surface and ten kilometers above the surface. Another consequence is that one cannot even calculate the temperature of the Earth without models that accurately reproduce the motions of the atmosphere. Indeed, present models have large errors here--on the order of 50 percent. Not surprisingly, those models are unable to calculate correctly either the present average temperature of the Earth or the temperature ranges from the equator to the poles. Rather, the models are adjusted or "tuned'' to get those quantities approximately right.
Richard S. Lindzen, Former Alfred P. Sloan Professor of Meteorology at the Massachusetts Institute of Technology.
To understand earth's temperature, we must also understand the role of water, water vapor, and ice in the earth system.
- The most important greenhouse gas is water vapor.
- It evaporates, mostly by the tropical ocean, in response to heating by the sun. Sunlight warms the ocean's surface, which cools by evaporation. In simple terms, the ocean sweats to keep cool. The water vapor then continues through the Earth's hydrological cycle.
- Some is carried into the Intertropical Convergence Zone (ITCZ) where it rises, condenses into rain, and releases the stored solar energy. Rain releases latent heat. This heats the air, drives the convection in the ITCZ, and it is the major heat source for driving the atmospheric circulation.
- The atmospheric circulation carries heat poleward, reducing the temperature contrast between poles and tropics.
- The circulation also carries water vapor high into the atmosphere, allowing it to radiate heat efficiently to space.
- Some condenses into puffy clouds. These clouds, and the convective clouds in the ITCZ reflect sunlight leading to a cooler earth.
- Some remains in the air and absorbs infrared energy emitted by earth. This increases the greenhouse effect leading to a warmer earth.
A major cause of concern is the relative importance of water in clouds and as vapor. Is the cooling by clouds more or less important than the warming by vapor? Water vapor and cloud drops can warm or cool earth's surface through feedback loops.
Aerosols
Small, microscopic, liquid or solid particles in the air (not the gas in aerosol cans), called aerosols, also influence earth's radiant energy balance. Aerosols reflect and absorb solar and infrared energy. Yet their importance is not well known.
Aerosols include:
- The water droplets that make up clouds.
- Soot (carbon particles) from forest fires and the burning of fields
and fossil fuels.

Thick smoke blankets the Southern California coastline as multiple brush and forest fires continue to burn near Los Angeles and San Diego. The Moderate Resolution Imaging Spectroradiometer (MODIS) sensor on the Terra satellite detected three major clusters of fires, marked with red boxes, on October 28, 2003.
From NASA Earth Observatory: Fires in Southern California. More recent images are at the Earth Observatory Fires web page.
- Volcanic emissions, including silicate microparticles and acid gases,
blasted into into the stratosphere by plinian
volcanic eruptions. The
year 1816 was known as the year without summer because so much sulphur
dioxide (which turned into small sulphuric acid aerosols) was thrown
into the stratosphere by the eruption of Tambora in 1815 that the aerosol
layer cooled the earth's surface by several degrees.
NASA has a short movie showing what happens.

Photographs taken by Space Shuttle astronauts about 24 hours after the start of the eruption of Rabaul Caldera. The eruption column rose to at least 18 km above sea level where the volcanic ash and gas were blown west to form a fan-shaped eruption cloud. A smaller eruption cloud (bottom photograph, lower right) was blown northward by lower-level winds.
From USGS Photographic Glossary: Eruption Cloud.
- Dust from dust storms on land.

A massive sandstorm blowing off the northwest African desert has blanketed hundreds of thousands of square miles of the eastern Atlantic Ocean with a dense cloud of Saharan sand. The massive nature of this particular storm was first seen in this SeaWiFS image acquired February 26, 2000 when it reached over 1000 miles into the Atlantic. These storms and the rising warm air can lift dust 15,000 feet or so above the African deserts and then out across the Atlantic, many times reaching as far as the Caribbean where they often require the local weather services to issue air pollution alerts as was recently the case in San Juan, Puerto Rico.
From NASA Visible Earth: Dust Storm. More recent images are at the Earth Observatory Dust and Smoke web page.
Additional Reading
- The University Center for Atmospheric Research has a simple tutorial on The Greenhouse Effect.
- For more information on the importance of CO2 and aerosols in the atmosphere, read the Executive Summaries for
of the Intergovernmental Panel on Climate Change IPCC Third Report on Climate Change 2001: The Scientific Basis. The IPCC was assembled by the United Nations Environment Programme and the World Meteorological Organization to provide the governments of the world expert advice on climate change and global warming. The Third Assessment Report follows similar reports issued in 1995 and 1989.
Reference
Naqvi, S. W. A., H. W. Bange, et al. (2005) "Biogeochemical
ocean-atmosphere transfers in the Arabian Sea." Progress
In Oceanography 65(2-4):
116-144.
Philander, S. G. (1998) Is the temperature
rising? The uncertain science of global warming. Princeton, Princeton
University Press.
Selby J.E.A., and R.A. McClatchey. (1975) "Atmospheric transmittance from 0.25 to 28.5 mm: Computer code LOWTRAN 3". Air Force Cambridge Research Laboratories, Optical Physics Laboratory Technical Report TR-75-0255.
Revised on: 29 May, 2017