2. General Remarks
LC phases can be divided into two classes. Thermotropic LC phases are formed by organic molecules in a certain temperature range and hence the prefix thermo-, referring to phase transitions caused by temperature change. About 1% of all organic molecules melt from the solid crystal phase to form a thermotropic LC phase before eventually transforming into an isotropic liquid at still higher temperatures. In contrast, lyotropic LC phases form a solution, and thus concentration controls the liquid crystallinity (hence lyo-, referring to concentration) in addition to temperature. Thermotropic organic molecules do not need solvent to form LC phase. The lyotropic LCs are formed by amphiphilic molecules in solution.
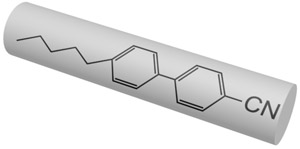
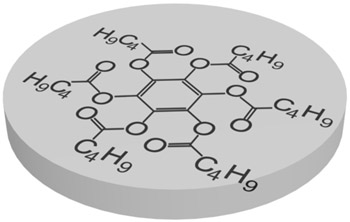
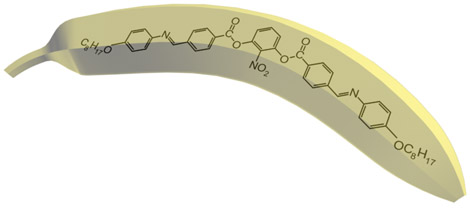
Nowadays there are a plenty of ways to generate a liquid crystal phase. The thermotropic LCs are usually formed by molecules with anisotropic shape, either elongated or disk-like. These molecules usually consist of a central rigid core (often aromatic) and a flexible tail (generally aliphatic groups). The elongated or rod-like molecules (Fig.1a) form calamitic LCs, and disk-like molecules (Fig.1b) form discotic LCs. The banana-shape molecule (Fig.1c) is an example of building blocks with more complicated shape, which form exotic thermotropic LC phases. The calamitic LCs are used in the most of practical applications.
|
(a) |
|
|
(b) |
|
|
(c) |
|
|
Figure 1. Molecular structure of 5CB (a), benzene-hexa-n-alkanoate derivatives (b) and banana-shaped (c) liquid crystals | |
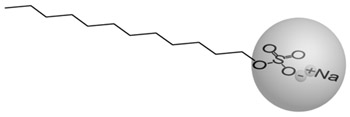
The lyotropic LCs generally are two-component systems where an amphiphilic compaunds are dissolved in a solvent. The building blocks forming lyotropic LCs are characterised by two distinct parts, a hydrophilic (can transiently bond with water through hydrogen bonding) polar head and a hydrophobic (repelled by water) nonpolar tail (Fig.2). Examples of these kinds of molecules are soaps.
|
|
Figure 2. Molecular structure of sodium dodecylsulfate (soap) |
Moreover, the LC phases can be formed by the high molecular weight molecules such as main-chain or side chain polymers composed from rigid mesogenic parts attached with flexible links.
The liquid crystal state (mesophase) exists within some temperature range, Tm<T<Tc, where Tm is temperature of melting from solid state into a mesophase, and Tc is clearing temperature, when the liquid crystal transforms into an isotropic liquid. In the solid state, the centers of gravity of molecules posses long-range positional order, and, also, the molecules orientation points in the same direction providing the long-range orientational order. When solid melts into a LC at Tm, the positional order is lost although some orientational order of the molecular long axes remains. At still higher temperature Tc, mesophase melts into an isotropic liquid with no positional and orientational order.
|
Figure 3. Phase diagram: Tm - melting temperature;
|
Prev
Introduction
Next
Director