Our research projects are very interdisciplinary and involve the synthesis and characterization of materials as well as knowledge of biological systems.
Research
A new trick for LC elastomers: Biocompatible, biodegradable, and porous liquid crystal (LC) elastomers a used for the first time as viable, soft scaffolds for cell growth and proliferation in a spatial (3D) fashion. These preliminary cell studies focused on characterizing the elastomer-based scaffolds’ biocompatibility and the successful 3D incorporation as well as growth of cells in 60 to 150-mm thick elastomer sheets.
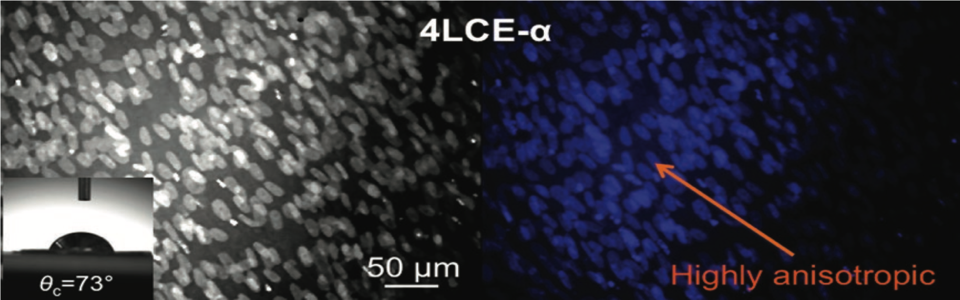
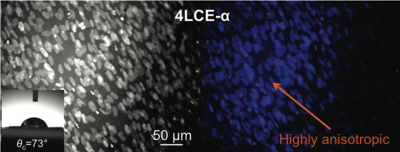
Biocompatible and biodegradable liquid crystal elastomers are investigated as spatial cell scaffolds, promoting cell attachment, proliferation, and highly anisotropic cell growth. Tuning the mechanical properties allows the creation of 3D cell cultures for a wide range of cell lines related to various tissues. Cell directionality tests are presented demonstrating that several LCE scaffolds show cell attachment, proliferation, narrow orientational dispersion of cells, and highly anisotropic cell growth on the as-synthesized LCE materials.
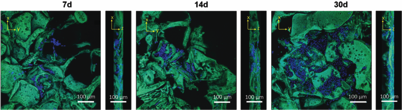
3D Porous Liquid Crystal Elastomer Foams Supporting Long‐term Neuronal Cultures, 3D liquid crystal elastomer (3D‐LCE) foams were used to support long‐term neuronal cultures for over 60 days. Retinoic acid was used to stimulate extensive neuritic outgrowth and maturation of proliferated neurons within the LCEs, inducing a threefold increase in length with cells displaying morphologies indicative of mature neurons. We showed that 3D‐LCEs provide a unique environment and simple method to longitudinally study spatial neuronal function, not possible in conventional culture environment.
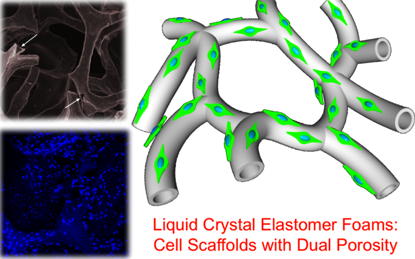
3D biodegradable and highly regular foamlike cell scaffolds based on biocompatible side-chain liquid crystal elastomers have been prepared. Scaffolds with a primary porosity characterized by spatially interlaced, interconnected microchannels or an additional secondary porosity featuring interconnected microchannel networks define the novel elastomeric scaffolds. The macroscale morphology of the dual porosity 3D scaffold resembles vascular networks observed in tissue. 3D elastomer foams show four times higher cell proliferation capability compared to conventional porous templated films and within the channels guide spontaneous cell alignment enabling the possibility of tissue construct fabrication toward more clinically complex environments.
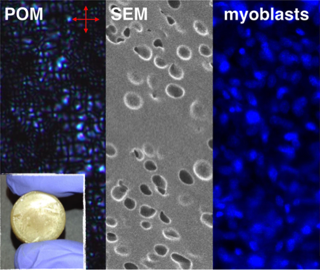
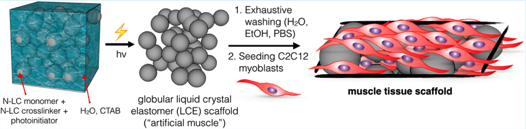
We report that liquid crystal elastomers (LCEs), often portrayed as artificial muscles, serve as scaffolds for skeletal muscle cell. A simultaneous microemulsion photopolymerization and cross-linking results in nematic LCE microspheres 10−30 μm in diameter that when conjoined form a LCE construct that serves as the first proof-of-concept for responsive LCE muscle cell scaffolds.
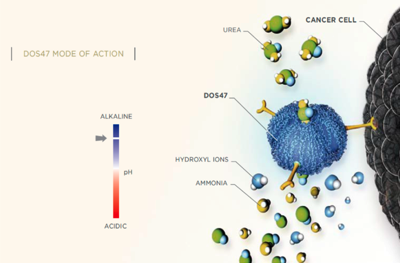
A novel immunoconjugate (L-DOS47) was developed and characterized as a therapeutic agent for tumors expressing
CEACAM6. The single domain antibody AFAIKL2, which targets
CEACAM6, was expressed in the Escherichia coli BL21 (DE3) pT7-7 system. High purity urease (HPU) was extracted and purified from Jack bean meal. AFAIKL2 was activated using N-succinimidyl [4-iodoacetyl aminobenzoate (SIAB) as the cross-linker and then conjugated to urease. The activation and conjugation reactions were controlled by altering pH. The specificity and cytotoxicity of L-DOS47 was confirmed by screening in four cell lines (BxPC-3, A549, MCF7, and CEACAM6-transfected H23). BxPC-3, a CEACAM6-expressing cell line was found to be most susceptible to L-DOS47. L-DOS47 is being investigated as a potential therapeutic agent in human phase I clinical studies for nonsmall cell lung cancer.
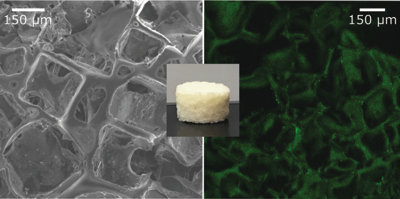
Liquid Crystal Elastomers (LCEs) have been long sought as artificial tissues based on a combination of orientational ordering, induced by liquid crystalline moieties, and elastic properties of polymers. Here we show a platform that provides a new insight into LCE–cell interactions where liquid crystalline materials promote cell alignment for appropriately stimulated tissue regeneration. Tissue regeneration requires 3-dimensional (3D) smart materials as scaffolds to promote transport of nutrients. We propose the use of responsive polymeric materials to create dynamic substrates for cell culture, which goes beyond designing only a physical static 3D scaffold. Here, we demonstrated that lactone- and lactide-based star block-copolymers (SBCs), where a liquid crystal (LC) moiety has been attached as a side-group, can be crosslinked to obtain LCEs with a porous architecture using a salt-leaching method to promote cell infiltration. The obtained SmA LCE-based fully interconnected-porous foams exhibit a Young modulus and a biodegradability rate optimized to mimic native environments. We present cell culture results showing growth and proliferation of neurons on the scaffold after four weeks. This research provides a new platform to analyse LCE scaffold–cell interactions where the presence of liquid crystal moieties promotes cell alignment paving the way for a stimulated brain-like tissue.


A Zero‐Power Optical, ppt‐ to ppm‐Level Toxic Gas and Vapor Sensor with Image, Text, and Analytical Capabilities, we created a zero‐power, optical toxic gas and vapor sensor platform for wearable or remote applications. Our sensor (acting as a dsiplay) gives a direct visual indication of dangerous levels of specific toxic gases and vapors. Our sensors are extremely economical to manufacture, and serve as warnings for specific uses for firefighters, military personnel in conflict zones, or employees in chemical manufacturing and water purification plants.


