The Carbon Cycle, the Ocean, and the Iron Hypothesis
What happens to the CO2 released into the atmosphere? An inventory of what is produced by human activity and what is stored in the atmosphere indicates that more than one half is missing. Part of the carbon goes into the land in the form of woody growth, but almost exactly 50% goes into the ocean (Sabine et al 2004).
Earth's carbon cycle is dominated by the ocean, which absorbs 50% of the CO2 released into the atmosphere by human activity. Carbon settling to the ocean bottom can eventually be stored for millions of years.
From Introduction to Climate Change United Nations Environmental Program's UNEP Global Resources Information Database (GRID) office in Arendal Norway.
The Oceanic Part of the Carbon Cycle
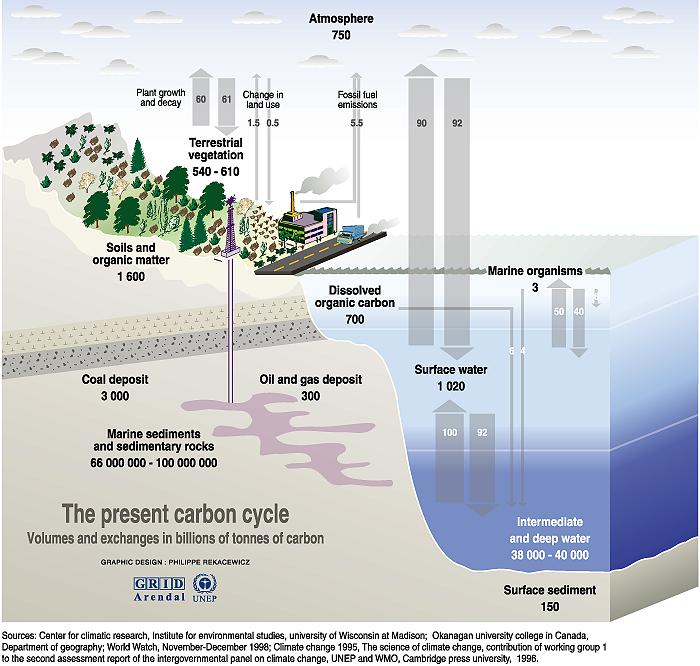
To understand the fate of CO2 in the atmosphere, we must understand earth's carbon cycle because atmospheric CO2 is only one part of the cycle. Several important oceanic processes influence the cycle. The figure above indicates that:
- The ocean stores 50 times more carbon dioxide than does the atmosphere;
- Much more carbon flows through the ocean than the amount produced by burning fossil fuels;
- An amount of carbon equal to to the total amount stored in the atmosphere cycles through the ocean in about eight years [(750 GT) / (92 GT per year) = 8.3 years]; and
- The flux in and out of the ocean is larger than the flux in and out of the land.
The carbon cycle in the ocean has two main parts, a physical part due to CO2 dissolving into sea water, and a biological part due to phytoplankton converting CO2 into carbohydrates.
- Carbon dioxide dissolves into cold ocean water at high latitudes.
CO2
is carried to the deep ocean by sinking currents, where it stays for
hundreds of years. Eventually mixing brings the water back to the surface.
The ocean emits carbon dioxide into the tropical atmosphere.
This system of deep ocean currents is the marine
physical pump for carbon. It help pumps
carbon from the atmosphere into the sea for storage.
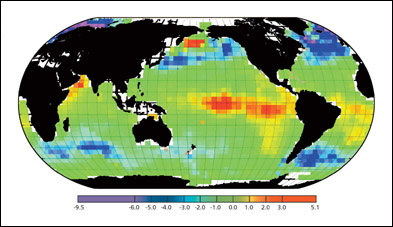
Global map of the average annual exchange CO2 flux (mol-C m-2 a-1) across the sea surface.
From Ocean Biogeochemistry and Global Change published by the International Geosphere Biosphere Program.
- Phytoplankton in the ocean use CO2, sunlight, water, and
nutrients and produce carbohydrates and oxygen. Animals eat the phytoplankton
contributing to the oceanic food web leading to fish. Dead phytoplankton
and animals sink deeper into the ocean, and some land on the
sea floor. The material from dead organisms is called reduced carbon.
It is carbon is that can be oxidized to yield energy, water, and CO2.
A small fraction of the reduced carbon (0.4%) is eventually buried
and stored in sediments for millions of years (Middelburg et al, 2007).
But most of the reduced carbon in and below the sea floor is used by
animals and bacteria, and returned to the deep-ocean part of the carbon
cycle. This is the marine biological pump for carbon. It
too pumps carbon from the atmosphere into the sea for storage.
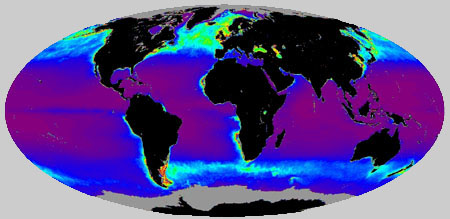
Global map of the primary productivity by oceanic phytoplankton. Click on the image for a different view.
From the International Geosphere Biosphere Program.
- The storage of reduced carbon in oceanic sediments in sediments
maintains the oxygen content of the atmosphere. If no reduced carbon
were stored in sediments, atmospheric oxygen would be used up in about
15 million years.
It's a popular misconception that the concentration of oxygen in Earth's atmosphere is controlled by photosynthesis. Photosynthesis is certainly the source of atmospheric oxygen, but the amount it produces is in almost perfect balance with the amount consumed through the respiration of living organisms. It is only when carbon-based matter is buried in ocean sediments, and so ceases to be decomposed, that atmospheric oxygen can accumulate. This burial process also reduces the levels of the greenhouse gas carbon dioxide released into the atmosphere. The exact rate of the burial of carbon-based matter is therefore a significant determinant of atmospheric composition, and thus global climate, over geological timescales.
From Masiello (2007).
- Animals in the ocean use carbohydrates and oxygen and emit CO2. Plants respire CO2 during the night. As a result, all the oxygen produced by phytoplankton is used to convert reduced carbon into carbon dioxide except for the small amount of reduced carbon stored in sediments.
- Recently, people started burning fossil fuels, which released, in the form of CO2, the carbon produced by plants and stored as reduced carbon (now in the form of coal, oil, and gas) in sediments millions of years ago.
Thus burning of fossil fuels is a source of CO2 and the ocean is a sink of CO2. To learn more about what happens to CO2 released into the atmosphere, read the paper on Sinks for Anthropogenic Carbon in the August 2002 issue of Physics Today.
Look at some images of chlorophyll distribution in the ocean to see where phytoplankton (microscopic floating plants) are common in the ocean. The Ocean Color home page has a nice animation of the seasonal cycle of phytoplankton concentration in the ocean. NASA Moderate Resolution Imaging Spectroradiometer team has produced a 6-year average map of chlorophyll concentration in the ocean.
Increasing the Oceanic Absorption of CO2: The Iron Hypothesis
If the carbon cycle in the ocean processes so much more carbon than does the atmospheric part, can the oceanic part be increased to cause the ocean to store more carbon? After all, a small change in the storage rate could absorb all the carbon dioxide released by the burning of fossil fuels. John Martin proposed a way to do this.
“Give me a half tanker of iron, and I will give you an ice age.”–John Martin.
Martin noticed that large areas of the ocean (30% to 40%) have sufficient nutrients to support the growth of large populations of phytoplankton, yet these areas have small populations of phytoplankton. He called these areas high-nutrient, low-chlorophyll zones (HNLCs). On further investigation, Martin determined the HNLC zones were deficient in iron, a micro-nutrient essential for life. Johnson then proposed that adding small amounts of iron to these regions would greatly increase productivity. This is the iron hypothesis.
Several recent experiments, including the Southern Ocean Iron Release Experiment, show the hypothesis is correct. Small amounts of iron in the right regions lead to larges increases in phytoplankton. One kilogram of iron leads to the production of 5,000 to 20,000 kilograms of phytoplankton.
Read about John Martin and his iron hypothesis, including all the information in links to his work shown on the right side of the web page. For a more controversial look at this solution to the CO2 problem, read the Wired Magazine article on Dumping Iron.
Increasing Land Absorption of CO2
Increasing the amount of carbon stored in biomass (forests) and soil reduces the build-up of carbon dioxide in the atmosphere. Freeman Dyson, in Heretical Thoughts About Science and Society notes that:
To stop the carbon in the atmosphere from increasing, we only need to grow the biomass in the soil, averaged over one half of the land area of the planet [the area used for crops and forests] by a hundredth of an inch per year. Good topsoil contains about ten percent biomass, so a hundredth of an inch of biomass growth means about a tenth of an inch of topsoil. Changes in farming practices such as no-till farming, avoiding the use of the plow, cause biomass to grow at least as fast as this. If we plant crops without plowing the soil, more of the biomass goes into roots which stay in the soil, and less returns to the atmosphere. If we use genetic engineering to put more biomass into roots, we can probably achieve much more rapid growth of topsoil.
Oceanic Phytoplankton
Most of the primary production in the ocean is by single-celled microscopic organisms. The organisms include:
- The Chromista, including Coccolithophorids, and Diatoms,
- Dinoflagellates. And,
- Photosynthetic bacteria and archaea.
To learn more about the micro-organisms in the ocean, read Marine Food Webs and Microbial Food Webs.
References
Masiello, C. A. (2007). Carbon cycle: Quick burial at sea. Nature 450 (7168): 360-361.
Middelburg, J. J. and F. J. R. Meysman (2007). OCEAN SCIENCE: Burial at Sea. Science 316 (5829): 1294-1295.
Sabine, C. L., R. A. Feely, et al. (2004). The Oceanic Sink for
Anthropogenic CO2. Science 305
(5682): 367–371.
Using inorganic carbon measurements from an international survey effort in the
1990s and a tracer-based separation technique, we estimate a global oceanic anthropogenic
carbon dioxide (CO2) sink for the period from 1800 to 1994 of 118 {+/-} 19 petagrams
of carbon. The oceanic sink accounts for [~]48% of the total fossil-fuel and
cement-manufacturing emissions, implying that the terrestrial biosphere was a
net source of CO2 to the atmosphere of about 39 {+/-} 28 petagrams of carbon
for this period. The current fraction of total anthropogenic CO2 emissions stored
in the ocean appears to be about one-third of the long-term potential.
Revised on: 29 May, 2017